

SONAS® Design Concept
Not for sale in the United States. For investigational use only.
Not for sale in the United States. For investigational use only.
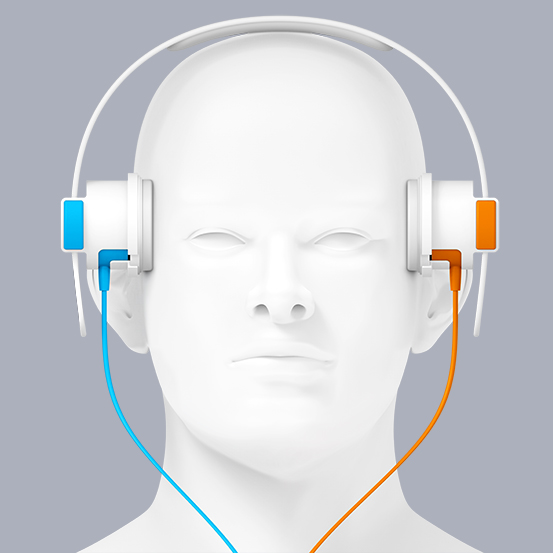
The concept of SONAS® is a portable, battery-powered ultrasound device for non-invasive brain perfusion assessment. SONAS® is used in combination with commercially available, approved microbubble contrast agents. Ultrasound transducers on each side of the head are installed in a headset. Following ultrasound microbubble injection via a peripheral vein brain perfusion is assessed in comparable fashion (right vs. left hemisphere) using proprietary signal detection algorithms and based on bolus kinetic modeling.
Key features
- Portable, battery-powered
- Utilizes commercially available, CE Marked ultrasound compatible microbubbles
- SONAS® consumables (including custom gel pads) are provided for each SONAS® test
- Simple user interface
- Results report generated in PDF file format
- Favorable safety (low output power)
- Cost effective


Technical Specifications
